PHYS 52 Lab - Green Sheet
PHYS 52 - Lab (Section 17) Thermal & Optical Physics
Instructor: Dr. Ray Kwok
Lab: Thurs 12:00 – 2:50 pm (Rm 133)
Office Hours: MW 9:20 – 10:20 am, 12:30 - 1:30 pm
Office: SCI 310
Phone: (408) 924-5252
Email: raymond.kwok@sjsu.edu
Course Description:
This lab section is complementary to the lecture on Thermal and Optical Physics. Fail to complete the lab section with a satisfactory grade will results an overall failing grade in the class. There will be an experiment every week. You'll need to have your data checked before leaving the lab. Lab reports are due a week after the experiment.
Required Materials:
- Scientific Calculator
- Spread Sheet (Excel)
Lab Report:
You are required to write up a report for each experiment. Group report is acceptable. Group size has to be 3 or less. However, fail to fully understand the experiment will cost you in the exams (see below). I do encourage paperless electronic report, however regular bonded lab book is acceptable. . If you choose to do electronic reports, please import your raw data and illustration of your set up by either scanning or photographing. All reports should be in a SINGLE file, submit to raymond.kwok@sjsu.edu with your names as part of the file name.
Your report must include the following:
- General info:
title, your name, data, course #, partner's name....etc - Introduction & Objective: (10%)
Any background information, purpose of the experiment, hypothesis, assumption...etc.
DO NOT just copy the brief statements from the info posted on the website. - Set-up:
A description of the experimental setup with a sketch, drawing or photograph. - Raw data: (20%)
Open your lab notebook and use it to record all information during lab. Do not use scratch paper, loose paper, the back of old envelopes, lab handouts, etc. to record lab information. All information should be recorded, in ink, in your lab notebook. If you make a mistake, draw a single line through the entry and make a note explaining the mistake. You never know, your original calculation may have been correct. Your data should be recorded in the order taken. You may wish to record run numbers (generated by the computer when collecting data), so you know which data in the computer corresponds with which run conditions from your experiment.
Start the lab by recording the date and the names of your lab partners.
Scan or take a photo of your raw data page & insert into your report. These pages should have my signature together with your name and date. - Data Analysis: (40%)
Show sample calculations. One calculation of each type is sufficient. Calculate percent difference from expected values whenever applicable: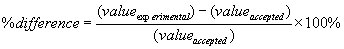
Include a summary chart. Arrange your results into a logical order in the summary chart in able to help you to spot trends in the data.
Any theoretical calculation, graph, curve fitting of the data, equations used, sample calculation...etc. should be presented. Make sure your tables, calculations, graphs, etc. have the correct units and labels. - Error Analysis: (10%)
List sources of errors in your experiment. Think about the experimental design (can some aspect of the experiment be improved to make the data more accurate?), random errors, and systematic errors. Do not use the “shotgun” approach of just listing every possible source of tiny error. Try to think of the one or two main sources of error. Does your data support your reasoning?
Note that systematic and random errors refer to problems associated with making measurements. Mistakes made in the calculations or in reading the instrument are not considered in error analysis. It is assumed that the experimenters are careful and competent!- Random errors are statistical fluctuations (in either direction) in the measured data due to the precision limitations of the measurement device. Random errors usually result from the experimenter's inability to take the same measurement in exactly the same way to get exact the same number.
- Systematic errors, by contrast, are reproducible inaccuracies that are consistently in the same direction. Systematic errors are often due to a problem which persists throughout the entire experiment. Example: your scale isn’t set up properly and reads 0.5 g too high for all of the measurements throughout your experiment.
- A quantitative error analysis should be presented whenever possible.
If x and y are 2 measurements with uncertainties Dx and Dy respectively, then the uncertainties add up for addition or subtraction of x and y, and the percentage errors add up for multiplication and division.- For example, if x = 10.0 +/- 0.1 (1%) and y = 20.0 +/- 0.1 (0.5%),
then, x + y = 30.0 +/- 0.2 (0.67%)
and x y = 200. +/- 1.5%
- For example, if x = 10.0 +/- 0.1 (1%) and y = 20.0 +/- 0.1 (0.5%),
- Conclusion:
Look for trends. If you change something, did it affect your results; did your results get better or worst? What could you do to improve the result if any.
The remaining 20% will be for the rest of the report as well as the quality of your work - experiment and write-up. The report reflects your understanding of the experiment and it should make sense to you years later when you look at it again. There will be questions on the mid-term and final exams to test your understanding of the experiments.
Make-up Policy:
Due to the nature of setting up equipment for the laboratories, there are no make-ups. A missed lab results in a zero for that lab. However, you may drop one lab from the computation of your overall laboratory grade.
Important Notes:
- You must pass the laboratory to pass the class. (50%)
- You must be in lab to collect your own data (even if you already had this lab in a previous class).
For More Information, contact:
Ray Kwok at SJSU
rkwok@sjsu.edu