Research Compliance
A key goal of the Office of Research is to ensure that research, scholarship and creative activities at SJSU are conducted safely, ethically and legally. The Office of Research facilitates the meeting of this goal by coordinating the University’s Institutional Review Board (IRB) and Institutional Animal Care and Use Committee (IACUC).
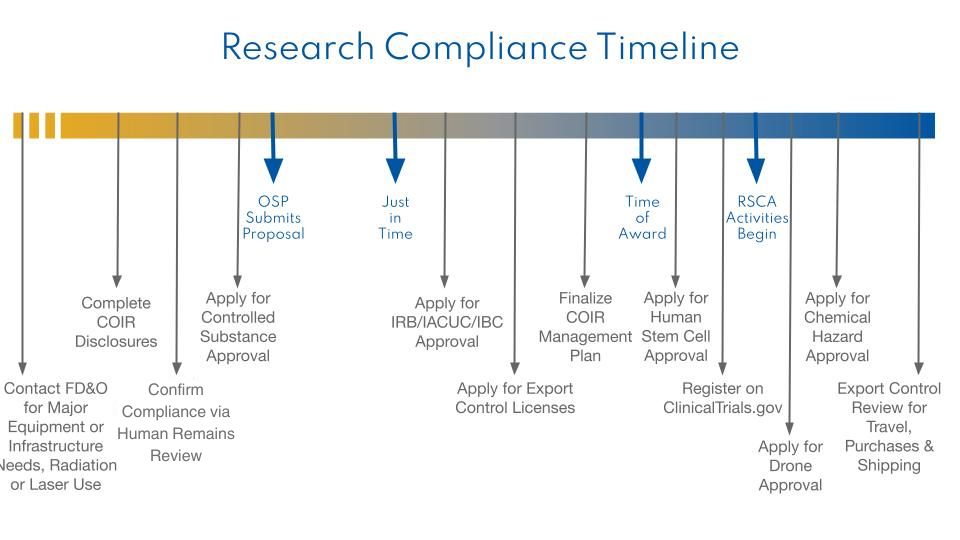
- Major equipment, infrastructure need, or radiation or laser use
-
Please contact the following individuals guidance as early in the grant writing process as possible:
For research that will involve lasers, contact the University Laser Safety Officer.
For research that will involve radiation, contact the Radiation Safety Officer.
If your research will require the allocation of additional space or involve the purchase of equipment that requires specialized installation (e.g. dedicated circuits or water lines) contact the DROs (Director of Resources and Operations) for your College.
If your research will require the renovation of your currently allocated space, contact Cynthia Soto in FD&O.
-
- Conflict of Interest in Research (COIR)
-
Your OSP Pre-Award Analyst should provide you with the necessary disclosures. If you have questions about completing these disclosures, contact the Research Compliance Unit.
-
- Human Remains
-
The procurement, use, transportation, and disposition of human remains or any other human materials in this category must be done safely and respectfully. Furthermore, certain remains may be subject to additional federal and California State regulations. If your RSCA activities involve human remains (whole body or body portion), human bones/bone fragments, or hair, teeth, or nails from deceased individuals you must contact the Senior Director of Research Services to confirm compliance with applicable regulations.
-
- DEA Controlled Substances and Listed Chemicals
-
EH&S Department is responsible for ensuring that research or experiments involving controlled substances or listed chemicals complies with Drug Enforcement Agency regulations. Prior to possessing or working with DEA controlled substances, a Controlled Substance Use Authorization application must be completed and submitted to EH&S. See complete lists of the U.S. Drug Enforcement Administration (DEA) controlled substances by schedule and the State of California list of precursor chemicals. Additional information and authorization applications can be obtained from the Chemical Hygiene Officer or the FD&O Lab Safety Page.
-
- Institutional Animal Care and Use Committee
-
Protocol forms are posted at the Institutional Animal Care & Use Committee (IACUC). In addition, all investigators and personnel working on a project using animals must complete the necessary Animal Health and Welfare Training. IACUC verification is required if the proposal is funded. If Animal Subjects are involved, you must contact Larry Young, College of Science, before you begin the research.
-
- Institutional Review Board
-
A protocol must be submitted to the Institutional Review Board (IRB). We recommend submission of the protocol package no later than sixty (60) days prior to the expected award date. Information regarding the use of human subjects is posted at the IRB website, located at Humans Subjects Research - Institutional Review Board (IRB). In addition, all investigators using human subjects must complete the web-based tutorial which can be accessed at Web-based human subjects tutorial videos. If Human Subjects are involved, you must contact the IRB Office before you begin the research.
-
- Institutional Biosafety Committee
-
The Institutional Biosafety Committee (IBC) is responsible for ensuring that recombinant DNA research or experiments involving biohazardous materials are conducted in compliance with NIH, CDC Guidelines, and Cal OSHA Requirements to promote safe and responsible work practices. Prior to possessing, storing, or working with or transporting infectious agents, select agents, and toxins, human or nonhuman primate materials (including blood, body fluids, cells, and tissues), recombinant DNA or transgenic animals, contact the IBC Chair to obtain approval.
-
- Export Control
-
Export Control Laws are a set of federal regulations that govern how information and technologies can be transferred internationally and prohibit certain activities without a license from the US Government. Purchasing research equipment, international travel, or sending data or technology to international collaborators can all trigger US export controls. Contact the Senior Director of Research Services to determine whether SJSU must apply for a license on your behalf.
-
- Human stem cells or embryonic tissues
-
If the project involves human adult stem cells (e.g. mesenchymal stem cells, hematopoietic stem cells, neural stem cells) or induced pluripotent stem cells (human somatic cells that are capable of dividing without differentiation in culture), the work requires review by a stem cell research oversight committee only if CIRM funds are being expended. If the project involves human embryonic stem cells, the research is covered by the California Health and Safety Code, Section 125119(a)(1) and must be reviewed and approved by a stem cell research oversight committee before you begin the research. Contact the Senior Director of Research Services to submit an application for stem cell research approval.
-
- Clinical Trials
-
All clinical trials sponsored by NIH must be registered on ClinicalTrials.gov and the consent form posted to a public federal government website. Furthermore, all individuals responsible for the design, conduct, or reporting of the clinical trial must complete training in Good Clinical Practice (GCP). You must contact the Director of Research Compliance to create an account on ClinicalTrials.gov.
-
- Drones
-
You will need to submit the make and model of the drone(s) being used, the plan for use, and any relevant licensing information to the Senior Director of Research Services, cc’ing SJSU Risk Management.
-
- Chemical Hazards
-
EH&S is responsible for ensuring that research or experiments involving regulated or listed carcinogens, acute toxins, or extremely hazardous chemicals are stored, handled and disposed of safely as required by local, state or federal regulations. Prior to obtaining hazardous chemicals, a Chemical Procurement Request Form must be completed and submitted to EH&S. You can find the form at https://app.docusign.com/templates by searching for "EH&S Chemical Procurement Request Form". The "single" version is for one product at a time, while the "multiple" version allows you to cover multiple products in a single submission.Then prior to working with the regulated chemicals, a Standard Operating Procedure (SOP) describing the storage, use, and disposal of regulated materials must be submitted to College Safety Staff or EH&S. Additional information can be obtained from the Chemical Hygiene Officer or the FD&O Lab Safety Page.
-
- International Travel
-
Traveling outside the United States on behalf of the University triggers additional insurance, IT security, and export control concerns. Visit the International Travel Information Page or contact the Senior Director of Research Services for more information.
-